TABLE OF CONTENTS
Introduction
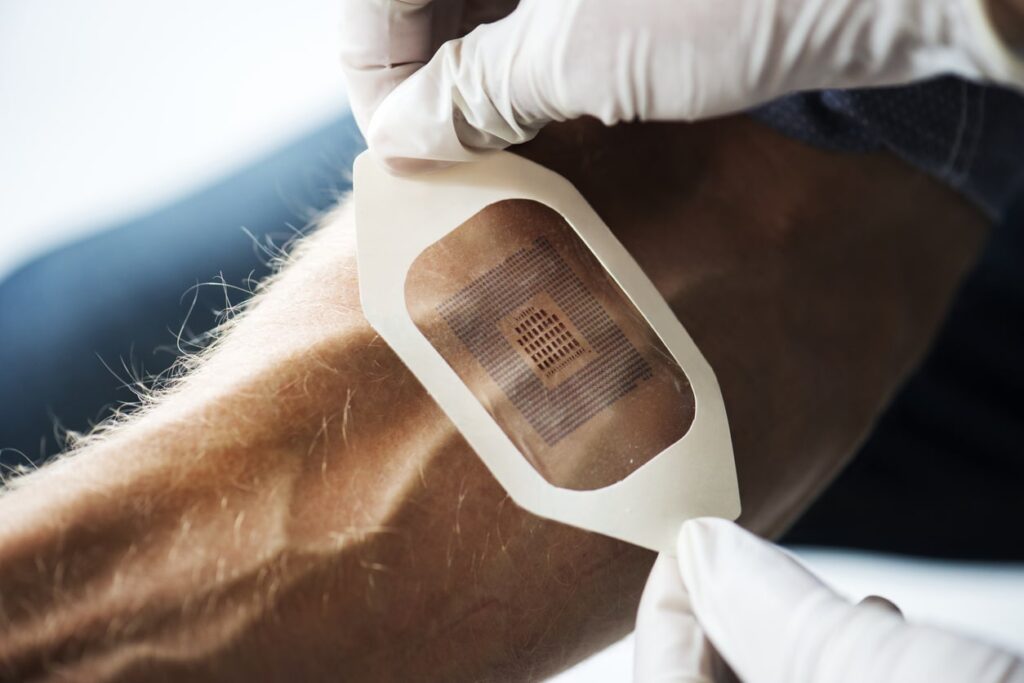
Not long ago, it seemed a farfetched idea that wearable devices would aid or even improve the way we approach healthcare. However, in recent years we have witnessed a rapid up-swing of such devices.
As of 2020, over 23 million patients in the US alone utilized some form of continuous and remote health monitoring and this number is expected to hit 30 million by the year 2024.
The sharp rise in demand for wearable medical tech has been fueled by its ability to measurably enhance patient monitoring, clinical decision-making, and exponential cost savings. Wearable technology is expected to save around $200 billion in global healthcare costs over the next 30 years.
And here’s the most important statistic.
Transparency Market Research (TMR) estimates that the medical wearable market will likely grow beyond $29 billion by 2026 at an expected 17% annual growth rate. Even a small percentage of this pie can be an extremely profitable proposition for the device maker.
While the market may be promising, developing a marketable medical wearable device is a monumental design and economic undertaking. There are several core fundamentals that manufacturers must consider so that their investment pays off.
The following sections will shed light on what these factors are and how to come up with a wearable product with commercial potential.
Designing A Stick-To-Skin Wearable For Scalable Production
A great design will amount to nothing if it cannot be manufactured at scale. Scalability is often one of the biggest challenges medical design engineers, medical adhesive suppliers, and converters face while translating a sleek idea into a commercially viable wearable medical device.
The Role Of A Seasoned Adhesive Materials Supplier In Ensuring Scalability
Partnering with an experienced medical converter and adhesive materials supplier will allow you to attain the best of superior design and scalable manufacturing that adds to your bottom line.
It is crucial for medical design engineers to work with experienced suppliers who can give honest and reasonable feedback so that both parties can agree on designs that can be mass-produced at an affordable price.
When a wearable medical concept proves viable and begins its journey to commercialization, it is vital that the medical device company and its supplier partners (adhesive material manufacturers, converters, and contract manufacturers) are ready to ramp up production.
Key Considerations Before Production
To ensure a smooth transition from prototyping to full-fledged production, the OEM and suppliers must address the following questions as early as feasible in product development:
- Can every component in the wearable design be procured in mass quantities at acceptable price points? As the demand grows for medical wearables, more players will enter the market and that means the margins will get slimmer. So you must ensure that the key design components are acquired at a fair price that doesn’t eat into your margins
- Do converting partners have enough capacity to meet forecasted demand? A well-designed wearable is no good if you can’t meet the demand. Make sure your converting partner has the capacity and technical expertise to deliver the wearables on time and within budget.
- Is quality always being considered throughout the supply chain to ensure patient/consumer safety? Sub-par quality will kill your business faster than a bullet! The modern-day customer is tech-savvy, knows what he wants, and is not shy about expressing their opinion. So you must work out an equation to deliver supreme quality at a commercially viable price point.
Suppliers must be willing to engage in frank discussions with OEM partners about their ability to scale from prototyping to producing large volumes.
Wearable medical device development is a dynamic and always-evolving area. There will always be the need for course corrections and fresh takes on device design. When medical companies partner with seasoned suppliers across multiple disciplines, they are much better equipped to move at the speed of digital health, staying a step ahead of the innovation curve.
Medical Adhesive Manufacturers & Medical Converters—Partnering For Optimal Material Selection In Stick-To-Skin Wearable Device Design
Medical device companies prefer partnering with suppliers and vendors who offer materials science expertise. Seasoned medical converters and adhesive material suppliers play a vital role in coming up with both creative product ideas and practical solutions for new stick-to-skin wearable concepts. They must work closely with suppliers to select the right materials for their wearable device.
Why They Matter
Medical adhesive manufacturers and converters often assist wearable device companies with product development. So, medical materials expertise and unrestricted access to a variety of adhesive and material samples give converters a clear competitive advantage. Let’s take a deeper look at the role they play in bringing the wearables to the market:
- Correct Material Selection: Wearable device companies look to medical adhesive manufacturers and converting partners to help them in selecting the right material for new wearable applications. Because of the intimacy between these devices and the patient’s body (20 minutes to over 3-week wear time), these wearables need to be constructed of skin-friendly adhesives and materials with extended-wear functionality.
A wearable device company will partner with a medical converter to seek advice on what materials will adhere best to a certain substrate or which roll is well suited for high-speed processing.
- Rapid Prototyping: The wearable device company may need a very quick turnaround on a prototype concept. Medical converters will want to develop relationships with medical adhesive materials vendors who offer wide product portfolios, including materials with many different thicknesses, sizes, configurations, and adhesive chemistries.
It is important for medical converters to have access to a variety of adhesive samples for quick prototype builds. It is not unusual for a medical device company to need a prototype within days or a few weeks at most. In such cases, the converter cannot afford to wait weeks for material delivery from suppliers.
Many medical converters have strong partnerships with adhesive material manufacturers and they stock samples at the medical converter for a quick turnaround in the development phases.
- Regulatory Compliance: The wearable device industry is growing steadily, and there is rapid expansion within the remote patient monitoring category. Wearable device companies need medical converting partners who are responsive and offer quick-turn prototypes. They also seek partners who comply with FDA and other regulatory requirements. Medical converters should collaborate closely with medical adhesive materials suppliers to help meet these needs.
Regulatory Considerations When Designing A Stick-To-Skin Wearable Device
No matter what the product, faltering on the regulatory front can be catastrophic for the manufacturer. The stakes are even higher when it comes to medical wearables because the wearer’s quality of life depends on them. Regulatory complacency will not only cause a loss of brand value and consumer trust but the manufacturer will likely have to face monumental legal challenges.
While we briefly touched upon regulatory compliance in the previous section, we’ll explore it in more detail here and discuss the key regulatory issues wearable device manufacturers must consider while bringing their products to the market.
Relevant ISO certifications
The greatest way to reduce cost and time to market when designing a stick-to-skin wearable device is to prioritize regulatory compliance and quality standards from the very beginning of product development.
From materials selection to prototype manufacturing, it’s advantageous and imperative to collaborate with suppliers who are ISO 13485 certified and compliant.
The designation should be considered with material science companies (adhesive manufacturers), converters manufacturing the adhesive tapes into usable parts for assembly, and the contract manufacturers assembling and packaging your final device.
This means their facilities’ quality management system meets rigorous standards for the design and manufacture of medical devices. ISO 13485 demands a thorough documentation process for all quality documentation record keeping as well as manufacturing and quality procedures in a manufacturing environment.
Additional Quality & Safety Considerations
In addition to ISO certifications, the manufacturers must ensure other quality and safety-focused features within the wearable supply chain. Here are some basic questions companies new to the wearable patient monitoring space should be asking potential material and manufacturing suppliers are:
- Does a supplier have clean room processing capability at both pilot-line and high-speed production levels?
- What level of clean room does the manufacturer use?
- Are sterilization services available?
While such questions may seem low on the priority list when compared with issues around data compatibility and wearable signal accuracy, they must be addressed to ensure patient safety.
In addition, when suppliers’ operations are registered with the appropriate regulatory agencies in the United States and globally, this will help streamline the path to regulatory approvals for the wearable down the road in the product launch process. When any major consideration affecting product quality and safety can be addressed sooner rather than later, the OEM stands to streamline wearable development.
How To Lower Cost & Time to Market for Medical Wearables
Gartner carried out a survey in 2019 and discovered that 45% of product launches are delayed by at least one month and roughly 1 in 10 businesses is not able to hit all of their internal launch deadlines.
And the medical tech space is no exception.
Considering how quickly the medical wearable market is evolving, it shouldn’t come as a surprise that more players are willing to invest and enter the market. This makes it imperative that OEM manufacturers take the appropriate steps to minimize their time to market and production costs to maintain their competitive edge.
We’ll explore here the key factors that could allow you to bring your wearable to the market at an accelerated pace while persevering your bottom line.
Seamless Component Procurement
We’ve mentioned it before and we’ll stress again, that a great design will amount to nothing if the components involved cannot be procured at scale or the right price point. This is the single most important factor that will bear on your wearable’s commercial viability and how quickly you can get it to the market.
Ensure Multi-Disciplinary Collaboration
Medical wearables is a fairly young industry. This makes it crucial that the key stakeholders are willing to share knowledge and resources. The willingness for multifunctional collaborations can considerably lower the overall production cost and accelerate the launch to market, therefore translating into a major competitive advantage.
Cross-functions partnerships allow the core stakeholders to quickly decide on the following core product aspects:
- What materials can deliver the best of form and functionality?
- What should be the ideal adhesion spot for the device on the body?
- How to minimize the tradeoff between wearable size, sensing capabilities, and battery life?
- How to accelerate the prototyping?
Being able to rapidly address these questions may shave months off the production process, thereby boosting the probability of the device’s market acceptance and commercial success.
What To Look For In A Reliable Converting Partner
Unquestionable credibility is the backbone of any enterprise that provides healthcare services and related products. This means your production and conversion partner must have extensive experience along with outstanding research and development capabilities. The Tape Lab checks all of these boxes. Let’s take a closer look:
Bulletproof Credibility & Extensive Adhesive-Specific Experience: The Tape Lab brings to the table over 19 years of research & manufacturing experience in the stick-to-skin medical space. Some of the largest names in the industry have entrusted us with developing adhesive solutions for their flagship continuous health monitor. We’re supremely confident we can meet and exceed your expectations as well.
A Wide Range OF Stick-To-Skin Products: The Tape Lab currently offers 11 individual categories for stick-to-skin products and 6 for wearable medical products. Our range includes but is not limited to adhesive bras, boob tape, Topical adhesive patches, fashion tape, wearable device tape, and diagnostic tape. You can explore our full product range here. No matter what you have in mind, you’ll find it under one roof with The Tape Lab.
The Ability To Customize & Unrivaled Engineering Expertise: Do you have an idea for a unique stick-to-skin product? We can help you bring it to life. The Tape Lab excels in every facet of medical adhesive design and manufacturing. Our core competencies include designing the tapes, die-cutting the patches, layer lamination, customizing the liner releases, and full-service OEM assembly. This gives us the scale and production capacity that will empower you to bring your product to market quicker and cheaper than your competition.
Strong Supplier Partnerships: What sets us apart is our rock-solid supply chain. We enjoy strong relationships with leading material manufacturers in the medical adhesive space. Our partners include 3M, Avery Dennison, Berry Plastics, and Polymer Science. This ensures immediate material access and enables us to design and produce customized medical adhesive solutions far quicker and cheaper than most of our competitors.
We Invite You To Book A Free Consultation
Looking for a full-service tape converter and adhesives specialist?
Call Us at +1 949-930-3112. – Our experts will schedule an obligation-free call and explain how we can put our full suite of converting services to work for you.
References:
- https://cardiovascularbusiness.com/topics/healthcare-management/healthcare-economics/medical-wearables-market-surpass-29b-2026
- https://blog.prevounce.com/27-remote-patient-monitoring-statistics-every-practice-should-know
- https://digitalhealth.folio3.com/blog/wearable-device-for-health-monitoring/#Wearables_Devices_and_Health_Care_Cost
- Prakash, Deepak. “Medical Device and Wearables Converting — a Primer and Update.” Medical Design & Outsourcing, 19 July 2016, https://medicaldesignandoutsourcing.com/medical-device-wearables-converting-primer-update/
- Prakash, Deepak. Reduce Cost and Time to Market for Medical Wearables. 29 Aug. 2016, https://mddionline.com/digital-health/reduce-cost-and-time-market-medical-wearables
- Gartner Survey Finds That 45% of Product Launches Are Delayed by at Least One Month. https://www.gartner.com/en/newsroom/press-releases/2019-09-09-gartner-survey-finds-that-45-percent-of-product-launches-are-delayed-by-at-least-one-month